Learning Outcomes:
i. Define chirality and identify chiral centers in organic compounds.
ii. Understand the concept of optical isomerism and differentiate between enantiomers and diastereomers.
iii. Recognize the properties of enantiomers and diastereomers, including their ability to rotate plane-polarized light.
iv. Apply the Cahn-Ingold-Prelog (CIP) priority rules to assign configurations to chiral centers.
v. Appreciate the significance of chirality and optical isomerism in various fields, including pharmaceuticals, biochemistry, and materials science.
Introduction
Chirality, a fundamental concept in stereochemistry, describes the property of an object that cannot be superimposed on its mirror image. In this lesson, we delve into the realm of chirality and optical isomerism, exploring the fascinating world of chiral centers and their implications in various fields.
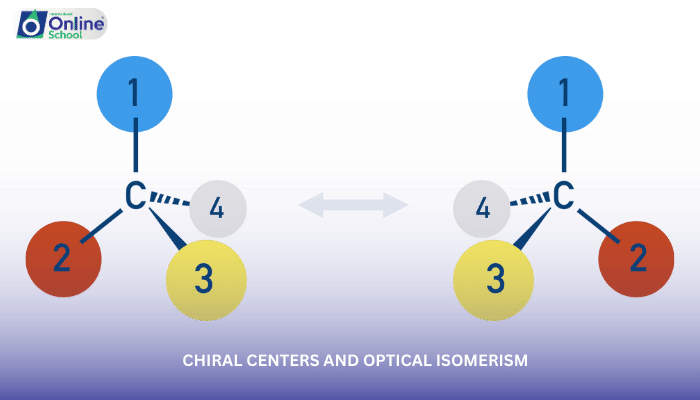
i. The Essence of Chirality
Chirality arises from the presence of a chiral center, a carbon atom with four different substituents attached. This tetrahedral arrangement creates a non-superimposable mirror image, resembling a right hand and its left-hand counterpart.
ii. Optical Isomerism: Mirror Images in Chemistry
Optical isomerism, a consequence of chirality, refers to the existence of non-superimposable mirror-image isomers. These isomers, known as enantiomers, have identical physical and chemical properties except for their behavior towards plane-polarized light.
Plane-Polarized Light: Plane-polarized light, with its electric field oscillating in a single plane, interacts differently with enantiomers. One enantiomer rotates the plane of polarization to the right (dextrorotatory), while its mirror image rotates it to the left (levorotatory).
iii. Diastereomers: Non-Mirror Image Isomers
Diastereomers are stereoisomers that are not mirror images of each other. They have different physical and chemical properties due to the different spatial arrangement of their substituents.
iv. CIP Priority Rules: Assigning Configurations
The Cahn-Ingold-Prelog (CIP) priority rules provide a systematic method for assigning configurations (R or S) to chiral centers. These rules assign priorities to the substituents based on their atomic numbers and the presence of double or triple bonds.
v. Significance of Chirality and Optical Isomerism
Chirality and optical isomerism have profound implications in various fields:
Pharmaceuticals: Enantiomers can exhibit different pharmacological activities, with one enantiomer being therapeutically active while the other may be inactive or even harmful.
Biochemistry: Many biological molecules, such as amino acids and sugars, are chiral and play crucial roles in life processes.
Materials Science: Chiral compounds can be used to develop materials with specific properties, such as liquid crystals and pharmaceuticals with controlled release rates.
Chirality and optical isomerism, arising from the presence of chiral centers, introduce a fascinating dimension to the world of organic chemistry. Understanding these concepts is essential for comprehending the behavior of chiral molecules, their biological significance, and their applications in various fields.